How about it, Hivers, the element oxygen is very likely to be the best known, the most studied and the most used by man, given its characteristics and utilities is what gives us energy to live and what our lungs capture to give the body the necessary functions as the fuel that moves us. Oxygen is a gaseous element that has certain slightly magnetic properties which does not have, smell, color or taste, but is responsible for giving energy through energy receptors (gills, lungs, etc.), aerobic organisms. It is the most electronegative element in the periodic table, perhaps only surpassed by Fluorine, which is highly reactive with most elements and is well known to oxidize very easily elements that have high electronic affinity. Oxygen is quite abundant in the Earth's crust and is the result of certain vital processes of other organisms such as plants and reefs, which within their biological processes the final result is the production of oxygen, in addition deposits of this gas are known in some areas of the poles of our planet, as well as it is known that it forms part of the fifth part in volumetric quantity of the air of our planet.[1-2-3]

Image Source
What is Oxygen for?
Oxygen, in spite of being that element known as the one that gives us life, but in spite of all this, this element enjoys an unparalleled amount of uses, due to its high reactivity. This non-metal, is capable of forming an enormous amount of substances, for its great electronegativity make it reactive to many elements except the noble gases. Many of the uses of oxygen have to do with the manufacture of steel, in the smelting and refining of this metal, where oxygen plays an important role in one of the stages of production, is used as an oxidant of some substances and then be removed to achieve the desired product, the great versatility in the use of this non metal, makes it also reactive to specific metals unwanted under strict control, in the production of steel. It is also used in the chemical laboratory for the elaboration of substances, under controlled conditions, in the oxidation of other elements in chemical synthesis, a process used on a large scale in the metallurgical industries. One of the most important uses of oxygen is as an oxidizer in the process of combustion of rockets, for the propulsion of these ships that carry material to space, due to its power and strength that provides the aerospace industry to have the specific mixture to lift large loads into space. Oxygen is mainly used in medicine, to treat people who require controlled assistance for some physical abnormality or disease, giving oxygen a good amount of uses, which cover several important aspects of the human being.[3-4]

Image Source
Did you know that Oxygen has three stable isotopes?
Oxygen has three stable isotopes that can be found in nature and the most abundant of them is O-16 which represents 99.7% in nature, besides being very flammable and reactive in any of its three isotopic forms, it has eight radioactive and unstable isotopes with a half-life measured in milliseconds and even nanoseconds. The second most abundant is O-18, which has an abundance of 0.2% and is used for the determination and study of water coming from outer space.[3-5]
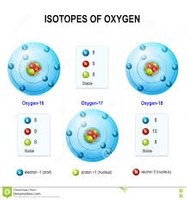
Image Source
Oxygen Curiosities.
There are three curiosities about oxygen that you probably didn't know and I'll name them below. On a geological level oxygen is a fundamental element for life, but oxygen is the most abundant element in the earth's crust and represents 46% of it, the rest is divided into other materials and minerals, in short the weight of the earth is mostly due to the amount of oxygen. Just as the earth's crust has large amounts of oxygen, so much so that we know that oxygen represents 75% of a person's weight, since we know that 90% of the body is made up of water and water has a very high proportion of oxygen, we can say that the but of your body is represented mostly by the amount of oxygen. Another of the curiosities of this element is the formation of the aurora borealis, where the solar energy reacts with the electrons that form the oxygen and these are responsible for the green and dark red color that can be observed in the aurora borealis, the atoms when losing energy produce these photons of visible light and it is for this reason that these phenomena of the nature can be observed, like complementary data, the auroras are formed thanks to our magnetic field, that protects us of the solar wind and when breaking this field these beautiful visual spectacles take place. [5]
Other uses of Oxygen.
Another use of Oxygen is in the manufacture of glass, where it is used to make different types of this material according to its oxygen concentration, there are materials of this type more resistant to heat and depending on the materials and amounts of oxygen, in addition to performing techniques necessary for the molecular rearrangement that gives the glass greater resistance. [4-6]

Image Source
Oxygen can also be found in the form of ozone, which is a fundamental material for life on earth, since it forms a protective layer throughout the planet to shield us from solar radiation. Ozone is a form of oxygen where three oxygen atoms produced under specific conditions are bound together. There are many other uses of oxygen that are well known given its reactivity and ability to form compounds, including inorganic compounds, such as the one exposed above for the production of glass.[3]

Image Source
In the reference area I will leave you more information to enrich your reading.

Disturbing note on Oxygen.
Although oxygen is vital, when the body is subjected to high pressure conditions, oxygen can be toxic to humans. Under diving conditions, divers who experience pressures greater than 50kPa can suffer oxygen poisoning, creating convulsions and even brain damage, which is why other gas mixtures are used under certain pressure conditions.
Recommendations.
If you are going to dive, always take care of the pressure to avoid oxygen poisoning; and remember that oxygen is highly reactive, so it is very flammable. If you are going to use oxygen in experiments or other activities, handle it with great caution and safety measures.

to carry out the translation into English you need the support of two translators for complex words or correct grammar: DeepL Traslate | Dictionry cambridge











Very nicely explained!
Thanks for your comment... @dlli999
@tipu curate
Upvoted 👌 (Mana: 147/250) Liquid rewards.
Oh! Thank´s @magicmonk