THE WORLD OF POLYMERS #3
THERMOPLASTIC AND THERMOSETTING POLYMERS
All the polymers discussed so far in my previous article have one property in common – they are thermoplastic. Thermoplastic polymers soften when heated and can be molded. The process of heating and molding can be repeated many times. At a molecular level, the forces between the polymer chains are weak, such as induced dipole-induced dipole forces or hydrogen bonds. These are sometimes referred to as secondary bonds. When a thermoplastic polymer is warmed, these forces are broken and the polymer chains can slide over one another, which allows the polymer to be shaped. When the polymer cools, the forces reform with the chains in their new positions.

This ability to be re-molded makes these polymers very versatile and therefore in much demand. Consequently, the annual world production of thermoplastic polymers is seven times that of thermosetting polymers.
Thermosetting polymers can be molded only when they are first produced. With strong heating, they decompose and cannot be softened and re-molded. A thermosetting polymer has covalent cross-links between its chains, so the polymer is a three-dimensional network of strong covalent bonds. On heating, these break, which causes the polymer to decompose before melting can occur.
BAKELITE: THE FIRST SYNTHETIC POLYMER
Leo Baekeland, a Belgian who had emigrated to the United States, invented the first wholly synthetic polymer, which he called Bakelite. He made it from the reaction of phenol with methanal. Bakelite was first manufactured in 1910.
The initial reaction involves a substitution in the benzene ring of phenol, which can be at either position 2 or position 4. Once formed, the product can react with another molecule of phenol with the elimination of a water molecule. The three-dimensional network is then built up through a series of similar reactions.

Ericsson Bakelite telephone, c. 1931. Holger.Ellgaard, CC BY-SA 3.0
As it has a high number of cross-links, Bakelite forms a hard rigid structure. Like most polymers, it is a good electrical and heat insulator. It was widely used in all types of articles, among them electric sockets and plugs, pan handles, and even music records, before PVC superseded it. But phenol-methanal resins are still important.
KEVLAR
Du Pont have been at the forefront of research on aromatic polyamides, called aramids, for a number of years. Kevlar is one of these aramids and is developed from monomers with functional groups in the 1,4 position on the benzene ring.
Kevlar polymer chains can be aligned to make very strong fibres. A 7 cm diameter steel cable has a breaking strain of about 40 tonnes. An identical Kevlar cable has the same breaking strain, but is five times lighter than the steel cable. Kevlar is embedded in tyres in place of steel reinforcement, which has reduced the weight of a typical truck tyre by 9 kg. However, the market for Kevlar-reinforced tyres has been slow to take off because tyre manufacturers have invested in equipment to produce steel reinforcement, believing that customers would prefer the strength that the word steel implies.
Kevlar is renowned as the protective padding in bullet-resistant vests. It is also incorporated into aircraft wings, in which strength combined with lightness is essential.

Kevlar vest. Cyril Thomas, CC BY-SA 3.0
TWO NATURAL POLYMERS: STARCH AND CELLULOSE
Natural polymers are mentioned in my previous post on polymers. Two other important polymers are the polysaccharides, starch and cellulose, both made from glucose monomers.
Glucose can exist in two distinct forms. Note the difference between these two glucose molecules by looking at carbon 1. This seemingly minor difference has a marked effect on the properties of starch and cellulose.
Starch is a polymer of α-glucose monomers. It is linked by α-1,4 linkages, also called glycosidic linkages. This form of starch is called amylose and is a straight-chain polymer. We digest starch by hydrolysing it with enzymes to give glucose. Starch is therefore an energy-storage compound, since once we have broken it down to glucose it can be oxidised in the body to give energy during respiration.
Starch can also be hydrolysed by acids in the laboratory to give α-glucose:
Cellulose, a linear polymer formed from β-glucose, is probably the world’s most abundant organic chemical. It has a structural role in plants and is the source of cotton fibre. In common with most animals, we cannot digest cellulose because we do not have the necessary enzymes to hydrolyse the β-1,4 linkage. However, cellulose forms the fibre we require in our diet.
WHAT DO WE DO ABOUT PLASTIC WASTE?
This problem will not go away, mainly because most of the polymers we throw away do not degrade (break down). In the UK, most plastic waste goes into landfill and that is where it will stay. So, what are the options?
Most polymers come from non-renewable crude oil, and it takes energy to produce them. In fact, of the energy that goes into making a plastic article, most is used in producing the polymer. So, why not recycle the polymer? The majority of polymers thrown out are thermoplastics, which could be melted down and reused. However, with some exceptions, all kinds of rubbish are thrown away together. It would take much energy and be fairly costly to sort it. Even if plastic waste were kept separate from other household rubbish.
The several different types of polymer involved would have to be further separated before they could be reprocessed. However, research is under way into cracking polymers, in much the same way as fractions of oil are cracked, to form a chemical feedstock from which new polymers can be produced. Plastic waste is also being cracked to produce petrol and diesel. But to be economically viable and environmentally friendly, any recycling scheme must not use up more energy than it takes to make the polymer in the first place.
Some articles themselves can be reused, which is achieved successfully for PET (polyester) bottles in some countries in Europe. Again, though, the energy consumption of recycling must also be taken into account.
Another way of dealing with plastic waste is to burn it and use the energy it provides. This is very controversial. Those who support this, point to the fact that most plastics do not biodegrade in landfill and it is better to at least extract the energy from them through incineration. However, opponents point environmental disadvantages of doing this. This is a non-renewable energy resource and it prevents recycling of plastic products so that they have to be made all over again from crude oil, expending considerable energy in the process and probably releasing carbon dioxide into the atmosphere. The very act of burning the plastic waste will produce more carbon dioxide, contributing to global warming and climate change. There are also real concerns about the toxic nature of some of the other emissions that occur during burning. Supporters of incineration point to the advances chemists have made in removing some of the toxic combustion products, such as hydrogen chloride from burning PVC.
DEGRADABLE PLASTICS
Chemists are playing a significant role in minimizing plastic waste by developing degradable polymers. One such polymer is poly(lactic acid)PLA. It has the advantage that it is made from renewable sources: corn or sugar cane. Bacteria ferment the sugar to produce lactic acid, which is polymerised to make the degradable polymer. PLA has found many uses from disposable cutlery and waste sacks to internal stitches in the body. There are two ways in which PLA degrades. The first is by hydrolysis linkage. This can be done by enzymes from microorganisms in the soil; this process is called biodegradation. The second is the absorption of radiation from light by the C=O bond, which splits PLA apart. This is called photodegradation.
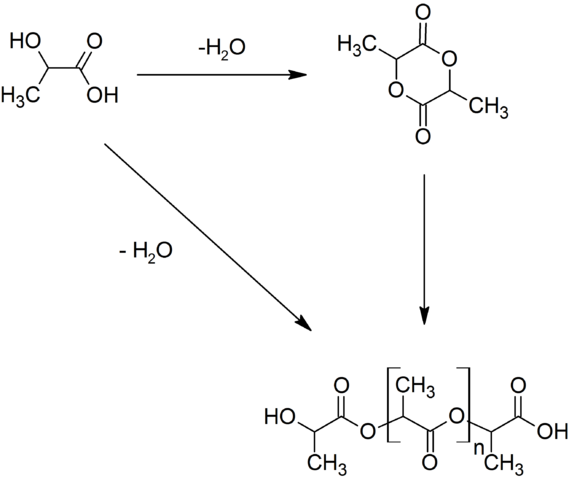
Generation of poly from lactic acid and lactide. Rifleman 82 - Own work, Public Domain.
Apart from biopolymers, there are other ways of making synthetic polymers degradable. For example, cellulose, starch or protein can be impregnated into plastic articles such as poly(ethene) bags. When such an article is buried in a landfill site, microorganisms break down the cellulose, starch or protein. The article then disintegrates. The synthetic polymer chains that remain have an increased surface area, which speeds up their decomposition.
Poly(alkenes) do not biodegrade in landfill sites if they are not impregnated with a substance that makes them biodegrade. However, polyesters and polyamides are hydrolysable at their ester and amide linkages. Enzymes from bacteria or acid catalysts will break the long polymer chains into shorter and shorter fragments, and so eventually they will biodegrade in landfill sites.
Another method with some polymers is to impregnate them with chemical activators so that on exposure to ultraviolet light the polymer chains break down into much shorter chains, which can then be biodegraded. Poly(ethene) can be made so that it will photodegrade. This is done by inserting carbonyl groups (C=O) into the polymer chain.
There are environmental problems even with degradable plastics. If polymers degrade, then the articles made of them may not be recyclable. Also, there is some concern over the unknown nature of the decomposition products, which may cause more long-term damage to the environment than the original plastics themselves.

Biodegradable plastic utensils. Scott Bauer, Public Domain
GENERAL CONCLUSION
The following are the final conclusion or summary of all the articles I’ve written on the world of polymers:
Polymers are very large molecules made up of many small molecules called monomers.
Addition polymers form when monomers react together without the elimination of small molecules.
Poly(ethene), PTFE, PVC (poly(chloroethene)) and poly(phenylethene) are all synthetic addition polymers.
Polymers such as poly(ethenol) are water soluble provided the number of hydrogen bonds is not too great or too small.
Polymers such as poly(propene) can be manufactured using Ziegler-Natta catalysts which use less energy and so are of great economic and environmental significance.
Condensation polymers are formed from monomers with the elimination of small molecules such as water.
Polyesters such as Terylene (PET) and polyamides such as nylon 6,6 and kelvar are synthetic condensation polymers.
Starch, cellulose and proteins are natural polymers.
Many synthetic polymers are not biodegradable and so environmental issues surround the disposal or recycling of plastic waste.
Condensation polymers can photodegrade by absorbing radiation at the C=O bond.
Finally, condensation polymers can degrade through hydrolysis at the ester or amide group.
REFERENCES
Bioplastics and biodegradable plastics
https://en.wikipedia.org/wiki/Biodegradable_plastic
http://www.chemistryexplained.com/Pl-Pr/Polymers-Natural.html
https://byjus.com/chemistry/natural-polymers/
https://sciencing.com/natural-polymers-8707376.html
https://www.cmu.edu/gelfand/education/k12-teachers/polymers/natural-synthetic-polymers/
https://en.wikipedia.org/wiki/Kevlar
https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/bakelite.html
https://www.youtube.com/watch?v=UiLMwDl1Y-4
https://en.wikipedia.org/wiki/Bakelite
https://www.modorplastics.com/plastics-learning-center/thermoset-vs-thermoplastics/
Hi, thanks for the post. I enjoyed reading about these polymers, especially the sections on bakelite and kevlar. I knew that kevlar was a strong material, but didn't realize that it was comparable to steel.
I included a link to your article in my recent post, Science and Technology micro-summaries for June 18, 2019, and set the beneficiary so that you'll receive 5% of the rewards from that post when it closes.
Thanks, @remlaps-lite. I do appreciate.
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having added @steemstem as a beneficiary to your post. This granted you a stronger support from SteemSTEM.
Thanks for having used the steemstem.io app. You got a stronger support!
Hi @empressteemah!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV