MOLECULES IN INTERSTELLAR SPACE AND A CLOSE LOOK AT ELECTRONS #2
Hello, my beautiful readers. Today, I will continue this article from where I stopped in my previous article on MOLECULES IN INTERSTELLAR SPACE AND A CLOSE LOOK AT ELECTRONS as promised. So, I’ll be starting my discussion on the atomic emission spectrum of hydrogen, followed by the Niels Bohr’s model of the hydrogen atom, etc.
THE ATOMIC EMISSION SPECTRUM OF HYDROGEN
When hydrogen is placed in a discharge tube at low pressure and with a high voltage between the two plates, some of the bonds in the hydrogen molecules (H2) are broken to give separate hydrogen atoms. When the radiation emitted from the discharge tube is passed through a spectroscope, a series of characteristic lines shows up in the visible spectrum.
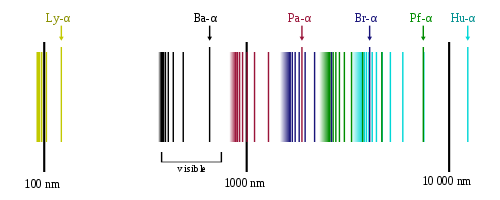
The spectral series of hydrogen, on a logarithmic scale. OrangeDog, CC BY-SA 3.0
THE BALMER SERIES
You read in my MOLECULES IN INTERSTELLAR SPACE AND A CLOSE LOOK AT ELECTRONS about Bunsen and Kirchhoff’s work in identifying elements by their characteristic spectral lines. These spectra are called line emission spectra for the following reason. When an element’s atoms are given energy, they absorb it and then emit radiation as discrete (separate) lines, at specific frequencies (and hence energies) for that element. For example, hydrogen, which has just one electron, has several prominent lines in the visible part of its emission spectrum. These are known as the Balmer series, after a Swiss music teacher who worked out a mathematical relationship between the lines.
OTHER SERIES
Other series of lines for hydrogen are found in different parts of the electromagnetic spectrum. The Lyman series is found in the ultraviolet section and the Paschen series in the infrared. Both these series are named after their discoverers.
Like the photoelectric effect, the different series of lines (characteristic for an element) was another 19th-century mystery that could not be explained by the theories of that time. An explanation had to wait for the ideas of Planck and Einstein to be developed.
ABSORPTION SPECTRA
Electrons absorb energy at specific frequencies, producing a series of black lines on a coloured background. This is called an absorption spectrum. The background is the visible electromagnetic spectrum, while the black lines are frequencies that are missing because electrons have absorbed these energies. The black lines occur in the same place as the coloured lines in the line emission spectrum, showing that electrons absorb radiation at specific frequencies and then release the energy at the same frequency.
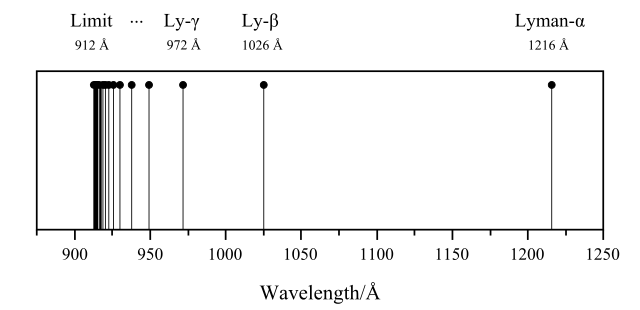
Lyman series of hydrogen atom spectral lines in the ultraviolet. Adriferr , CC BY-SA 3.0
USING THE LIGHT OF ELECTRON TRANSITIONS
Neon is used everywhere in advertising signs. An electric current is passed through the gas at low pressure. The fast-moving electrons of the electric current excite electrons in the neon atoms into higher energy levels and, when they return to lower energy levels, orange-red light is emitted. The colour can be varied by adding other atoms such as argon or mercury, or by colouring the glass tube the neon is in.
Street lights usually contain sodium or mercury and they work on the same principle as neon lights. When excited mercury atoms return to their ground state, the radiation they emit has frequencies in the ultraviolet, yellow, green and blue parts of the spectrum.
Sodium lights have replaced mercury ones across the UK road network because the radiation emitted by excited electrons in sodium atoms when they return to their lower levels is centred upon yellow. This has longer wavelengths than the light from mercury and is not as readily scattered by fog, so it can illuminate further. Also, sodium atoms require less energy to excite their electrons, and sodium is not as toxic as mercury.
Fluorescent lights and low-energy light bulbs in the home or office contain low-pressure mercury vapour. The inside of the lighting tube is coated with a phosphor, which absorbs the energy of ultraviolet light when its electrons are excited. On returning to the ground state, these produce many frequencies of light in the visible range, which combine to give white light. A substance fluoresces when it takes in light of one wavelength, usually outside the visible spectrum, and gives out light of another, often in the visible spectrum.
The advantage of fluorescent lights and low energy light bulbs over filament lamps is that they use less energy, since nearly all the energy is radiated in the visible spectrum. They feel cool to touch, while ordinary light bulbs with tungsten filaments waste energy as they become very hot. However, they do contain up to 50 mg of mercury, which is an environmental hazard when they are thrown away.
The Lyman series of lines is found in the ultraviolet part of the spectrum. It is caused by the excited hydrogen electron returning from higher levels to the n = 1 ground-state energy level. As this is the lowest level nearest to the nucleus, far more energy is released when an electron excited to a particular energy level returns n = 1 than when it returns to n = 2, and so the lines show up in the more energetic ultraviolet part of the spectrum.
Each line of hydrogen’s emission spectrum represents one electron transition: a movement from a higher to a lower level. As large numbers of hydrogen atoms are involved, all the possible transitions are represented, which gives the full spectrum of lines.
NIELS BOHR’S MODEL OF THE HYDROGEN ATOM
In 1911, we were left with the Rutherford model of the atom. However, there are problems with this model. Rutherford proposed that electrons orbited the nucleus, rather like planets round the Sun. Planetary motion was well understood by this time – the Sun’s gravity pulls planets towards it, while their acceleration, caused by being in a circular orbit, creates a balancing force. (For an object to travel in a circle, it is constantly changing direction, so it has to accelerate constantly.)
Negative electrons in circular motion are attracted to the positive protons in the nucleus by electrostatic forces. If they orbited the nucleus like planets, their acceleration would keep them from falling into the nucleus. But electrons are charged particles, and accelerating charged particles were known to emit electromagnetic radiation and lose energy. If Rutherford’s model was correct, instead of a few separate lines, a continuous spectrum should have been observed, with the atom emitting light all the time and the electron losing its energy and falling into the nucleus, causing the hydrogen atom to collapse. Clearly, this does not happen!
Another model of the atom was needed to explain the observations. Niels Bohr used the quantum ideas developed by Planck and Einstein to propose his model for the hydrogen atom. Bohr’s model still had the hydrogen electron orbiting the nucleus. But the orbits, or energy levels that the hydrogen could occupy were quantized – that is, they had fixed energy values. With this model, hydrogen’s line emission spectrum could be explained.
In Bohr’s model, the electron normally occupies the lowest possible energy level, called the ground state. This is the energy level of the electron when it is not excited. Raising the electron to an excited state (giving it energy), say by an electric discharge, causes it to move up to a higher level by absorbing a quantum of energy. When it returns to the lower, ground state energy level, it releases this quantum of energy as a photon of light of a specific frequency, so giving a line in the emission spectrum.
Imagine that the hydrogen electron is like a ball on a staircase, the ball can rest on any step, but it cannot stop in between. This is the case with the electron. The ball needs energy to go up the step, and when it falls back down it releases this energy. The lines in an emission spectrum are closer together at one end because the higher energy levels that the electron can occupy are also closer together. This happens as the electron moves away from the nucleus.
When the electron is closest to the nucleus, it is at its lowest energy level. Moving the electron away from the nucleus requires energy, and the further away it is moved, the more energy it requires. So the more energy an electron receives, the higher it can rise through the energy levels, and the more energy it will release as it falls back down again.
Each energy level is given a number, called the principal quantum number, n. The term ‘principal quantum number’ is still used to describe the main energy levels of electrons in an atom. When the hydrogen’s electron is in the n = 1 level, it is not excited, so this is the ground state.
The Balmer series of lines is for the energy transitions when the excited hydrogen electron falls back from higher energy levels to n = 2. We see the lines because they are in the visible spectrum. The higher the energy level the electron falls from, the higher the frequency of the emitted photon. Note that the energy levels eventually merge at n = α (infinity). This is when the atom has become ionized and lost its electron, which has escaped from the nucleus’s attraction.
IONIZATION ENERGY (IONIZATION ENTHALPY)
From the Lyman series we can work out the energy needed to remove an electron completely from a hydrogen atom. This is the ionization energy for hydrogen. It is the energy required to take the electron from the ground state, at n = 1, to where the energy levels converge at n = α, when the electron is free of the attraction of the nucleus.
SUMMARY OF THE BOHR MODEL OF THE ATOM
- Electrons exist only in certain permitted energy levels and in these levels they do not emit energy.
- Electrons move to higher energy levels by absorbing quanta of energy. They return to lower energy levels by emitting these quanta as photons of light, which show up as lines in different parts of the electromagnetic spectrum.
The Bohr model of the atom successfully explained the lines in the emission spectrum of the hydrogen atom. It worked for hydrogen, the simplest atom with just one electron, but it did not predict accurately the spectral lines of atoms with several electrons.
The Bohr model is important because it used the idea of quantized energy levels to explain atomic structure and provided a foundation on which others could build. The Nobel Prize went to Bohr in 1922, one year after Einstein had received the prize for his explanation of the photoelectric effect.
REFERENCES
http://www.ece.utep.edu/courses/ee3329/ee3329/Studyguide/ToC/Fundamentals/Bohr/description.html
https://www.kentchemistry.com/links/AtomicStructure/waveenergy.htm
https://study.com/academy/lesson/electron-transitions-spectral-lines.html
https://web.phys.ksu.edu/vqmorig/tutorials/online/hydrogen/emission.html
https://brilliant.org/wiki/energy-level-and-transition-of-electrons/
https://en.wikipedia.org/wiki/Emission_spectrum
https://users.physics.ox.ac.uk/~smithb/website/coursenotes/qi/2016_QI_Lecture_4-6.pdf
https://www.toppr.com/guides/physics/atoms/atomic-spectra/
https://www.chemguide.co.uk/atoms/properties/hspectrum.html
https://courses.lumenlearning.com/introchem/chapter/emission-spectrum-of-the-hydrogen-atom/
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch6/bohr.html
Atomic structure and periodicity
https://en.wikipedia.org/wiki/Hydrogen_spectral_series

Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having added @steemstem as a beneficiary to your post. This granted you a stronger support from SteemSTEM.
Thanks for having used the steemstem.io app. You got a stronger support!
Hi @empressteemah!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Thanks again for this nice post about the foundations of quantum mechanics :)