[English Version] | 💡WHAT HAPPENS TO Phosphorus💡 Uses and curiosities // Get to know a bit of science in style and very simply
SIMPLE SCIENCE

¿What about Phosphorus?

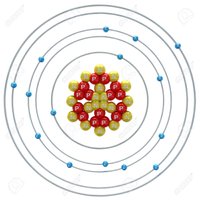
Greetings hivers of science, we arrive today at the fifteenth element of the periodic table, which we are reviewing since fourteen posts and today it touches the Phosphorus, which is a very abundant chemical element in the terrestrial crust and that is fundamental for the life in our planet. Of symbol P, the phosphorus is a fundamental element for the processes of transference of energy and the important compound in this process are the phosphates, being these the ones in charge of the photosynthesis, metabolisms of the enzymes, nervous functions and activity of the muscles that thanks to these compounds allow the effective functionality of these so vital processes for the life. Phosphorus is a highly reactive element, to the point of being freely reactive in the atmosphere, that is why it is not found isolated in its pure state, but forming other compounds and it is very common to find it present in the apatite mineral. This is an element that has different allotropic varieties among which are white phosphorus, red phosphorus and black phosphorus.[1-2-3]

What is the Phosphorus for?
Phosphorus has a large number of applications in various industries, among them, it is known that the use of phosphorus compounds, phosphates and others, are used in the fertilizer industry as one of the essential components that fertilize the soil and thus give the necessary nutrients to plants, giving agriculture good distribution products. In its allotropic forms, white phosphorus is used for the manufacture of pesticides, among which are the development of rat poison and also in the manufacture of fireworks and explosives, due to its incredible reactivity in the presence of fire. The red phosphorus, is used in the manufacture of matches that are also known to carry the name of the same element, this system is simple but very essential and in many cases what has the phosphorus is what the head of the match, which is based on sulfur is rubbed to be lit. Another application of phosphorus is in the metallurgical industry, where it is used in alloys for the reinforcement of other metals or to give characteristic properties to some metal.[2-3]

Phosphorus, monostopic element
Despite the fact that 23 isotopes are known to them, only one is stable and corresponds to 100%, which is P-31, with the longest half-lives being P-33 for about 25 days and P-32 which has a half-life of about 15 days. The rest of the unstable phosphorus isotopes have half-lives measured up to nanoseconds, these being discovered only under strict laboratory studies. [4]
Curiosities of Phosphorus.
Among the curiosities that this element has, is that its discoverer called it "Cold Fire" because after isolating it he discovered that being in absence of light it has a chemiluminescent shine, since it produces a green light, this motivated to that the white phosphorus reacts with the oxygen of the air, causing this interesting effect. Among other curiosities, there is that the allotropy of this element shows us several colors of phosphorus, where the best known are white and red phosphorus and are the most common, but also are the violet and black. Red phosphorus is one of the chemical elements that are used in the manufacture of methamphetamines. [3-5]
Other uses of Phosphorus.
Among the other most common uses of phosphorus is the manufacture of animal feed and additives for certain drugs, of which phosphorus is the active element within the needs of the formula. [2-3]
It is also used for the manufacture of additives in plasticizers, coatings for metal surfaces, which are necessary for their protective coating and subsequent transfer and additives in petroleum products. [1]

In the reference area I will leave you more information to enrich the reading.

Disturbing Note on Phosphorus.
Despite being a necessary element for the human body, but the consumption of white phosphorus is very harmful to health causing severe burns on contact with it and if ingested, can cause serious problems in the kidneys, liver and heart, even if not treated in time can lead to the death of the affected person.
Recommendations.
In spite of being a friendly element for the human being he must take his precautions at the moment of manipulating some compounds of Phosphorus.

| Referencias | Link |
|---|---|
| 1 | Phosphorus |
| 2 | Properties of Phosphorus |
| 3 | Phosphorus definition and characteristics |
| 4 | Phosphorus Isotopes |
| 5 | Curiosities about phosphorus |
| 6 | 10 curiosities of the Phosphorus |
to carry out the translation into English you need the support of two translators for complex words or correct grammar: DeepL Traslate | Dictionry cambridge

0
0
0.000
0 comments