[English Version] | 💡WHAT HAPPENS TO NEON💡 Uses and curiosities // Get to know a bit of science in style and very simply
SIMPLE SCIENCE


What about the Neon?

What's up, Hivers. Let's know another of the elements belonging to those known as noble gases, this is an inert gas and known to be in this group of elements that do not react with other elements in normal conditions of pressure and temperature on our planet. This element has 10 electrons which means that it has all its molecular orbitals full, where its two orbitals S and its orbital P, are full making that there is no space for the rearrangement of other electrons in its external layer, to form compounds. This element, in spite of being the fourth most abundant element in the universe, is very scarce in our planet being found in the air in only 0.018%, this is extracted by fractionation of the air through subcooling and later distillation of the liquid, from the monoatomic gas. This is an odorless, colorless and tasteless gas which is well known to be used in the manufacture of lamps and lasers. The chemical element Neon was discovered in 1898 by Sir Ramsay.[1-2-3]

What is the Neon for?
Neon gas is commonly used for the manufacture of lamps, many of them for advertising, since in contact with the electrical current it generates a great illumination. The color that generates this gas is reddish orange, which is very common to see in advertising lamps, although there are lamps of other colors, they are called "Neon lamps", but these are generated by other noble gases. Liquid neon has also been used as a refrigerant, where in spite of being more difficult to obtain than helium, it is more viable in some cooling systems. [4]

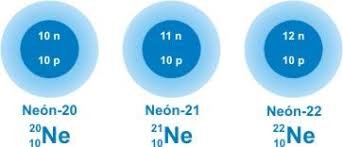
Neon has three stable isotopes
Neon has a long list of isotopes but only three are stable which are Ne-20, Ne-21, Ne-22. Among the radioactive isotopes only Ne-24 has a half-life of 3 minutes, the rest do not exceed one minute of half-life. Ne-20 is the most abundant isotope with a 90% abundance and the second isotope is Ne-22, with a 9% abundance being the most used. [1-5]
Neon Curiosities.
Among the curiosities of the Neon, is that it is the most susceptible to the electric current, since its discovery in 1898, this element has fascinated thanks to its luminous form, besides providing a good environment for the study of nuclear particles, where the space inside the tube for the detection, is exclusive for the neon, extracting all the air and replacing it with neon. In the study of particles, among other laboratory studies that require an effective detector of charged particles, this element is the most suitable for this purpose. Neon really has some limited uses and in this sense its use is specific since for other activities, other elements can be used that are more abundant, in spite of this it is a well known element. [5-6]
Other uses of Neon.
The neon is used as a high voltage indicator thanks to its susceptibility to voltage, having an intensity depending on the amount of current flowing through it, this is why it makes it a very good material as a high voltage detector. It is also used in lightning rods that function as a protector and at the same time a detector of the amount of current. [4-6]

Another of the main uses where Neon is in the manufacture of television tubes as well as in obtaining a type of laser when it is in a mixture of gases with helium. [3]

In the reference area I will leave you more information to enrich your reading.

Disturbing note on the Neon.
if the gas is inhaled in closed places without good ventilation due to an oversaturation of this gas can generate asphyxiation. Liquid neon can also be dangerous on contact with the skin or any mucous membrane, because it is a freezing element and contact with any part of the body can cause the area to freeze and generate serious burns.
Recommendations.
In the liquid state it must be worked very carefully, as it can cause burns.

| Resources | Link |
|---|---|
| 1 | Neon |
| 2 | Chemical Properties of Neon |
| 3 | Neon, what is the chemical element Ne used for? |
| 4 | Neon Uses |
| 5 | Neon Isotopes |
| 6 | Neon data and curiosities |
to carry out the translation into English you need the support of two translators for complex words or correct grammar: DeepL Traslate | Dictionry cambridge

0
0
0.000
@tipu curate
Upvoted 👌 (Mana: 120/150) Liquid rewards.
Upvoted 👌 (Mana: 105/150) Liquid rewards.
Thank´s @magicmonk