Water- A Solvent like no other
As I progress through my dissertation's laboratory synthesis method, I've come to respect and better grasp how vital solubility/dissolution and water are to us as living things, and how everything around us lives as it does. This blog will simply provide a few fascinating points to consider regarding water's dissolving capabilities!

Source-Pixabay
Turpentine, ethanol, and sulfuric acid are excellent at dissolving items. Paint, oil, metal, skin, bones, and more can all be broken down by them! However, there is another liquid that can dissolve more chemicals than anything else on the planet: WATER, sometimes known as "The Universal Solvent." Even still, this powerful dissolver can be found anywhere as it rains down from the heavens, filling our pipes. We use it to bathe, wash our hands, and pour it down our tender throats.
This raises an intriguing question for me: if water is such an effective solvent, why doesn't it dissolve everything?
Water is excellent in dissolving a wide range of substances. To understand how a liquid dissolves something, we must first understand that it must pull apart and keep the molecules that make up that item apart. Simply put that water was made for that purpose! Its molecules have significant positive and negative charges, which allows them to pull apart and get between other molecules or molecules with charged parts.
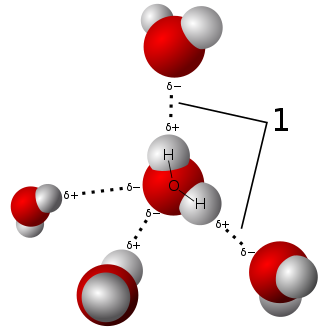
1.Water Molecules pulling apart ; Source ; Public Domain
Interestingly, many different molecules have charged parts, which means that many different chemicals on our planet dissolve at least to some level in water, and thus water is the preferred cleaning substance. It can remove sticky fruit juice from your counter as well as blood from your clothes :) Water, on the other hand, does not disintegrate the counter, the clothes, the windows, or the streets! Pretty interesting, don't you agree?
Water's incredible dissolving abilities, on the other hand, are precisely why our planet is built the way it is. Because water cannot dissolve these materials, we make our countertops out of quartz, our clothes out of cotton, our windows out of glass, and our roadways out of asphalt. They're usually made up of molecules with no charged components. It would be absurd to construct windows out of sugar, for example (something that can dissolve in water). Many of the compounds that water dissolves, such as washable markers, are those that we've designed to be wiped away. When we don't want them to be washed away, we've created variants that aren't dissolvable in water.
We've been able to adjust our lives to a world where water dissolves so much stuff by making sure water dissolves what we want it to dissolve. Humans have physically adapted to a planet where water dissolves so many things; Our skin's outer layer is constructed in such a way that it is uncharged and thus insoluble. Similarly, the membranes that wrap each of our cells contain an insoluble layer. The only reason we humans, or trees, fish, microbes, or anything else exist on this watery planet is because we evolved barriers to block water from dissolving us into mush. Those that didn't make it are simply dead in the water.
Realizing that our planet's existence is dependent on water's incredible dissolving power has been sort of an eye-opener for me! Shouldn't we cherish it more, having in mind that only about 1% of all the water we have is actually useful for us.
Thank you for taking the time to read through! Please share your opinions in the comments section! Have a great time buddies!
Check out more here, if you like :)
Why Is Water the Universal Solvent?
Solvent properties of water
Water
Solvent Molecules
Posted from HypeTurf
As I read this article I remembered a moment in high school chemistry. I was an OK chemistry student, but the class at that moment was completely disinterested in the lesson. The teacher was talking about how fats don't dissolve in water. It hit me. When I baked cookies and heated, water the butter dissolved. Why? The whole class broke out in laughter, although I was quite serious, and the teacher was relieved. I woke them up! She explained (this was almost 60 years ago) that when water is heated the molecules move more rapidly and because of that they more freely engage with fat molecules (or something like that). Here I am again, sixty years later, reading about dissolving things in water.
On a more serious note, it's a great lesson for teachers, I think. If we make something real and relevant then people will listen (are more likely to listen). I think you make stuff real in your article. Thank you for the walk down memory lane :)
Glad i brought back some sweet memories from the good ol days :)! Communicating scintific topics to be comprehensible by anyone should be the goal of every teacher and making it realistic as much as possible goes a long way as you have rightly suggested. Thanks a lot for reading through!
:)
Great Post!
!1UP
Thank you!
You have received a 1UP from @luizeba!
@stem-curator, @vyb-curator, @pob-curator, @neoxag-curator, @pal-curatorAnd they will bring !PIZZA 🍕
Learn more about our delegation service to earn daily rewards. Join the family on Discord.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Aaaahhh water :)
I agree with @agmoore's comment. This blog is a great way to shed light on water as a solvent.
On an almsot non-related note, I also played a bit with water this year to illustrate simple electrostatic properties. As water is an electric dipole, we can make flowing water react to charged Ebonite (hard rubber) or glass sticks. Look for "Bending water with static electricity" online. You can find really cool stuff ^^
Cheers!
Thanks for reading through! Will sure look into this! Perhaps i could master the trick to since i've always wanted to be a "water- bender" haahaa! 😁
I did in live in front of 50 students. There is definitely no trick. It is pure electrostatic attraction/repulsion ;)
Awesome! The beauty of science! :) I trust your students enjoyed watching you do it as much as you enjoyed doing it :)
They usually like a lot when we run small experiments in front of them ^^
We also build our lectures through an app, so that we can ask questions live, and see what they answer (how they would predict the results of an experiment for instance). This is very cool to make the course interactive.
Sounds super cool! They get to enjoy from the very best... You should do a recording of these experiments and share with us as well, if you can :)
Maybe next year. This year was the first one and everything was somewhat experimental, if you see what I mean... ;)
Yeah sure! That is absolutely fine.. definitely looking forward to it