Chemistry of Glass "Part 3": Properties of Glass.
Window glass and bottles are produced using glass that is made from sand, lime, and soda. If the sand contains iron oxide, the glass will turn green because of ferrosilicon, and the hardness of the glass may be increased by substituting part of the sodium with potassium.
The glass used in optical lenses has a high lead oxide content. Pyrex, is used in the production of laboratory tools, has a lower calcium and sodium oxide content than regular glass but contains borosilicate oxide in place of silica. As a result of its extremely low coefficient of thermal expansion, Pyrex is a borosilicate glass that resists heat.

Measuring cup
Thermal expansion:
The thermal energy "E" increases as the temperature "T" gets higher, which causes the particle vibration amplitude to go up as well.
E= K. T
K: Boltzmann constant= R/NA
NA: Avogadro's number= 6.0221408e+23
R: Ideal gas constant= 8.22 J/mol. K
Thermal expansion happens as the temperature rises, causing the distance between particles that are force-bonded to one another to expand.
αΔT= (1/ l0).(Δl/ΔT)
αΔT: Average coefficient of increase in length (Coefficient of linear expansion)
l0: Initial length
Δl: Length variation
ΔT: Temperature variation
βΔT: Average volume increase coefficient (Coefficient of cubical expansion)
βΔT= (1/V0). (ΔV/ΔT)
V0: Initial volume
ΔV: volume variation
In general: β= 3α
Viscosity:
The term "supercooled liquid" refers to glass. Glass may be described as a highly viscous liquid. The following graph represents the viscosity curve f(T):
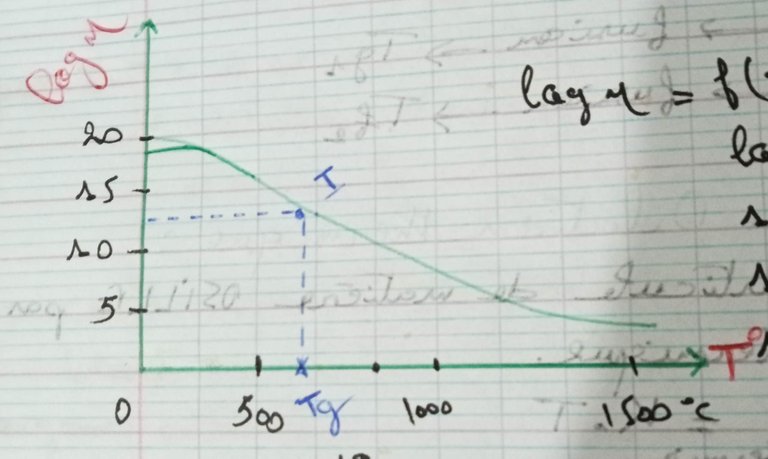
[Self made]
At room temperature η= 10 to the power of 19 poise. Log η drops and η decreases as the temperature rises. The log η equals 2 when the typical melting temperature for calcium silicate glass, Tf= 1500°C.
The temperature Tg required for the glass to freeze (solidify) is T=500 °C (log η=13). (Log η= 2 _ a melting _ at T= 1500 °C).
Elasticity:
Under the influence of a deformation force, a solid body is deformed. An elongation described by the elastic modulus arises under tensile stress:
E= l/Δl. F/S
E: Young's elongation coefficient
l: Rod length
Dl: The elongation undergone by a rod
S: section
F: Strength
Hardness:
By using the Vickers pyramid and a diamond imprint, hardness is determined. Vickers microhardness Hv=1.854=P/(d squared).
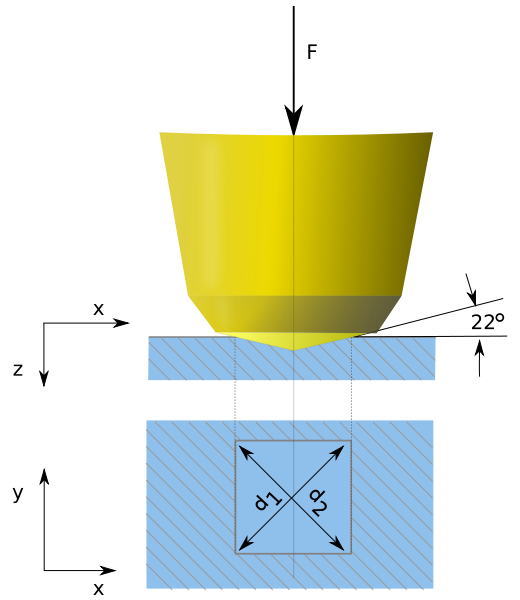
Schematic of a Vicker's hardness test
P: Load applied
d: Diagonal of the imprint

Pyramidal diamond anvil impactor on a Vickers hardness tester
Density:
The specific Density of a calcium silicate glass is 2.5.
Tensile strength Rt:
The minimal point at which a hung glass rod (of sectional area 1 millimetre square) breaks indicates the tensile strength. In general Rt= 5 Kg.f/mm square. Cylindrical rods that are a few centimeters in length and a few millimeters in diameter are used for the measurement.
Compressive strength Rc:
For glass, the resistance to compression is very high, Rc = 20Rt; Rc = 100 Kg.f/mm2. Because of the powerful Si-O interactions that occur in silicon dioxide, this high value is achievable.
Chemical Properties:
Hydrofluoric acid (HF) causes a noticeable assault, it makes glass dissolve.
SiO2+ 6HF ==== H2SiF6+ 2H2O.
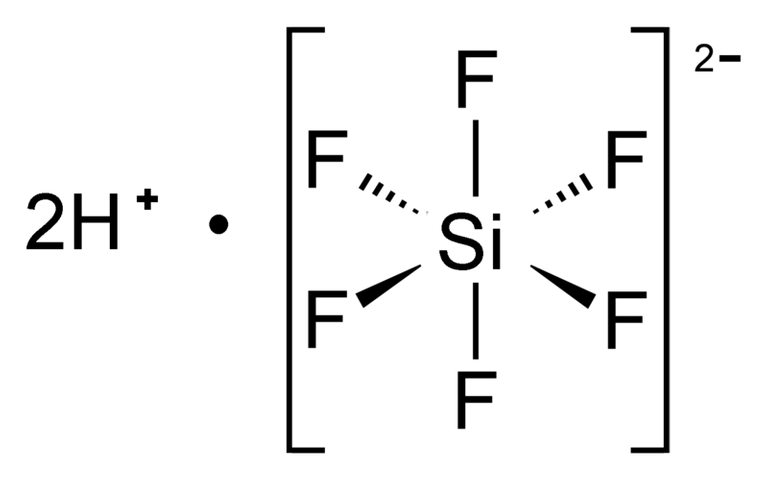
Hexafluoro silicic acid H2SiF6 molecular structureThe vitreous network breaks down when the OH- ion reacts with it in alkaline conditions, which might result in the creation of soluble silicic acid ions. It denotes a glass dissolution.
References:
- [General and inorganic chemistry book- M. Shkhashirou- H. Birqdad- Y. Qodsi- University publications. Algeria]
- Glass and Ceramic Technology Course (2021). Professor Khelifa- Department of Materials Process Engineering- University of Mostaganem. Algeria.
- Les matériaux au cœur du processus d'innovation- Clefs CEA No 59.
- Neumann, Florin. "Glass: Liquid or Solid – Science vs. an Urban Legend". Archived from the original on 9 April 2007. Retrieved 8 April 2007.
- Helène Tregouët. Structure et cristallisation de verres d’oxydes simples riches en bore et en terres rares. Chimie-Physique [physics.chem-ph]. Université Pierre et Marie Curie- Paris VI, 2016. Français. NNT: 2016PA066032. tel-01358710.

Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
I learned a lot from this one. Thanks
I appreciate that, thank you for your time :)