Chemistry of Glass
Glass is a crucial component of the civilisation we live in today. Without it, we would not have had telescopes, the microscope, or the discoveries made via it, and we would still have windows with wooden shutters that block light from the rooms. Without it, the only source of illumination would be a candle.
There are many stories about the appearance of glass for the first time in some civilizations, but it is not certain when exactly glass was invented. This invention is often attributed to some Phoenician sailors who noticed a glassy substance formed when they were lighting a fire on sand and placing their pots on stones of natron (natural sodium carbonate), as this glassy substance was formed from the union of natron, silica and potassium salts (present in the ashes). In addition, Glass vessels from 3500 BC were discovered in certain Egyptian tombs, and their chemical makeup is quite similar to that of flask glass today.
From a chemistry point of view, Glass is an inorganic substance that cools from a liquid form and transitions immediately to a solid form with no crystallization. And therefore, Glass is a non-crystalline, amorphous substance.
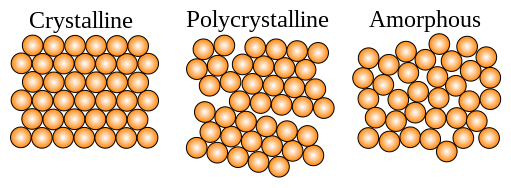
Schematic of how atoms are arranged in crystalline, polycrystalline, and amorphous matter.
Vitreous state:
When a liquid is abruptly cooled and the atoms or molecules that make up the liquid do not have time to assume their equilibrium position, the resulting solid substance is said to be vitreous or amorphous, and is disordered, which implies that its structure lacks symmetry and regularity.
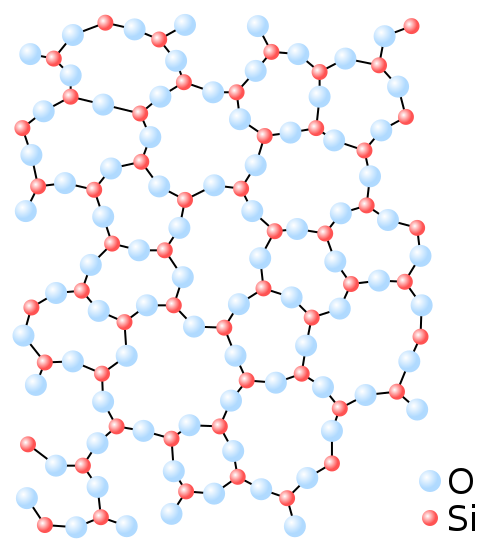
The amorphous structure of glassy Silica (SiO2) in two-dimensions.
Some properties of the vitreous state that distinguish it from the crystalline state:
- Neither a melting nor a solidification point exist.
- without the coexistence of two phases, a continuous (gradual) transition from the liquid state to the vitreous state.
- There is a set temperature of change of state at a particular pressure.
To be continued.....
References:
- [General and inorganic chemistry book- M. Shkhashirou- H. Birqdad- Y. Qodsi- University publications. Algeria]
- Glass and Ceramic Technology Course (2021). Professor Khelifa- Department of Materials Process Engineering- University of Mostaganem. Algeria.
- Les matériaux au cœur du processus d'innovation- Clefs CEA No 59.
- Neumann, Florin. "Glass: Liquid or Solid – Science vs. an Urban Legend". Archived from the original on 9 April 2007. Retrieved 8 April 2007.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.